
15 Residual Elements In Steel
2023-07-26 10:38The problem of residual elements in steel is one of the important problems faced by the metallurgical
industry. During the steelmaking process, steelmaking raw materials (including molten iron, scrap steel
and ferroalloy, etc.) will bring a large amount of impurity elements into the steelmaking furnace. Some
of the impurity elements can be removed, but some of the impurity elements will remain in the steel.
This part of the impurities (alloy elements not intentionally added) are collectively referred to as residual
elements.
These residual elements are one of the main factors for the instability of steel quality. Certain residual
elements are prone to segregation and even at low levels can have a strong negative effect on steel
properties.
Such as residual titanium in bearing steel is a typical case. Ti is easy to react with N to produce
high-hardness inclusions, which greatly affects the service life of bearing steel.
1. Classification of residual elements
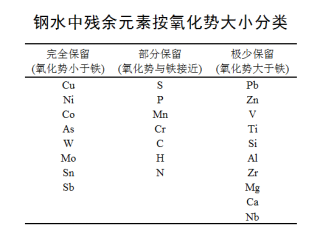
The known residual elements in steel are divided into three categories according to their oxidation potential,
as shown in the following table. They are shown as complete retention, partial retention and minimal
retention in the steelmaking process.
In the above table, the oxidation potential of the first type of elements is lower than that of iron, and
they do not participate in the oxidation reaction during steelmaking, and almost all of them are
eventually accumulated in steel products.
The oxidation potential of the second type of residual elements is close to that of iron. During the
blowing process of steelmaking, only a part is oxidized and removed, and the degree of removal is
related to the characteristics of the elements themselves.
The oxidation potential of the third type of elements is higher than that of iron. During the blowing
process of molten steel, they are first oxidized into the slag for removal, and only a small part of thementers the product.
Therefore, the problem of residual elements in steel is actually only 15 elements contained in the first
and second categories. Among them, 8 kinds of elements are fully reserved elements, and 7 kinds of
elements are partially reserved elements.
2. The source of residual elements in steel
my country is a country with many symbiotic iron ores, which include V, Ti, P, As, Sn, Sb, Re (rare earth
elements), etc., which are brought into steel during smelting.
In addition to the residual elements brought into molten iron by primary iron ore, the largest source of
residual elements in molten steel is scrap steel, which is mainly divided into:
(1) Alloy steel in scrap steel. At present, there is no cost-effective technology for steel mills to sort alloy
steel and ordinary carbon steel, and some medium and high alloy steel contain a wide variety of alloy
elements. In the recycling of steel, these alloying elements will enter the steel as residual elements;
(2) Surface coating or plating in scrap steel. The most problematic one is tinplate, which enters the scrap
steel cycle as a can box. Other coatings include copper, nickel, and chromium, etc.; galvanized sheets are
also widely used, but zinc can be basically removed in steelmaking without consideration ;
(3) Non-ferrous metals wrapped in scrap steel raw materials. The most important thing is automobile
scrap steel, which contains some micro motors, and the main impurity is copper.
On the market, copper has the most residual element content, and copper is mainly entered into
steelmaking furnaces from automobile scrap steel. It is estimated that the average copper content in
mixed steel scrap in steel mills is about 0.3%, and the specific content depends on the source and
proportion of alloy steel.
The residual Sb and As in steel mainly come from primary iron ore. When scrap steel containing these
impurities enters recycling, they can be diluted, but the residual amount will gradually accumulate in
steel.
The H and N in steel mainly come from the workshop atmosphere during steelmaking, and their
content mainly depends on the composition of different steel grades and steelmaking process.
3. Segregation of residual elements in steel
Many residual elements exist and function in the form of segregation in steel. Most residual elements
have strong segregation ability in steel; the segregation process of this element can occur not only in
the solidification process of molten steel, but also in the subsequent solid phase transformation, but it
takes a long time for diffusion.
The main segregated elements in the riser part of the ingot are S, P, and C, followed by Sb, N, As, H,
and Sn elements. After segregation forms inclusions, the hardness of this part of the material is also
higher than that of other parts of the ingot.
Compared with solidification segregation, residual elements will produce grain boundary segregation
during solid phase transformation or heating. For example, the second type of temper brittleness of
steel is mainly caused by P, Sn, As, and Sb grain boundary segregation.
4. Brief description of the role of residual elements
① 8 kinds of fully reserved elements
Ni, Co, W, Mo can improve the hardenability of steel and are beneficial elements;
On the one hand, Cu can cause copper embrittlement during high-temperature thermal processing of
steel, but on the other hand, it can improve the ability of steel to resist atmospheric corrosion;
Residual elements Sn, As, and Sb are harmful elements, which not only strengthen copper brittleness
in steel, but more importantly, it will cause the second type of temper brittleness of alloy steel;
Sn is one of the extremely harmful residual elements in steel, and Sn will greatly reduce the
high-temperature mechanical properties of steel and alloys.
② 7 partially reserved elements
C, Mn, S, P are conventional control elements;
Cr can improve the oxidation resistance of steel, increase the corrosion resistance and hardenability of
steel, but also increase the temper brittleness of steel;
N is beneficial to control the grain size of austenite, but at the same time it will also cause the strain
aging of steel;
H in steel is a harmful and unhelpful element, which can cause white spots, cracks in low-allohigh-strength steel, etc.
